Introduction
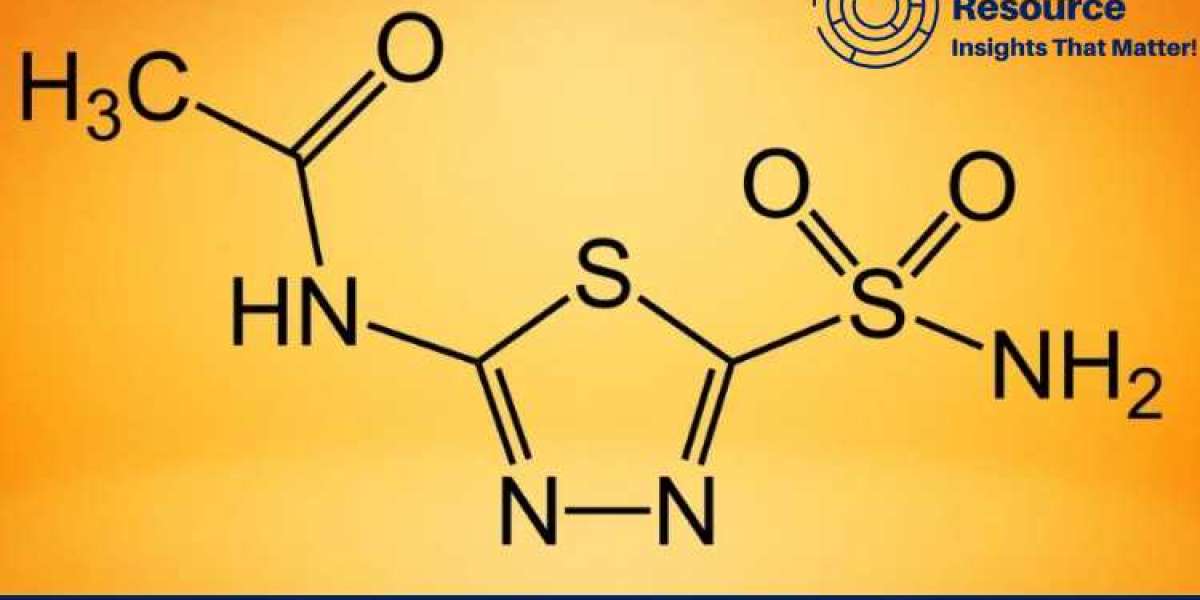
Acetazolamide (Diamox) Production Process with Cost Analysis is a critical framework for understanding the complex process behind manufacturing this important pharmaceutical product. Acetazolamide, commonly known by its brand name Diamox, is a carbonic anhydrase inhibitor used primarily to treat glaucoma, altitude sickness, epilepsy, and edema. The production process of acetazolamide involves various chemical synthesis stages, precise quality control measures, and significant resource allocation. This report delves into the acetazolamide production process, procurement resource assessment, market drivers, raw materials requirements, and cost analysis, providing valuable insights for manufacturers and industry stakeholders.
Request Free Sample – https://www.procurementresource.com/production-cost-report-store/acetazolamide/request-sample
Procurement Resource Assessment: Acetazolamide (Diamox) Production Process
The production of acetazolamide requires a highly coordinated procurement strategy that ensures the availability of raw materials, energy, and equipment necessary for efficient manufacturing. The procurement resource assessment for acetazolamide production includes:
Raw Material Sourcing: The key raw materials for acetazolamide production include sulfur, acetic acid, ammonia, and specific organic intermediates. These materials must be of pharmaceutical-grade quality to meet stringent regulatory standards. Ensuring a stable supply of these inputs is essential to avoid disruptions in the production process.
Technology and Equipment: Acetazolamide production involves several steps, including sulfonation, condensation, and crystallization. The production facilities must be equipped with advanced reactors, filtration systems, and drying units. Automated technologies for process control and monitoring can improve efficiency and product consistency while reducing human errors.
Energy Management: Chemical synthesis and purification processes, particularly during condensation and crystallization, require significant energy. Effective energy management, including heat recovery systems and optimized cooling and heating cycles, can help reduce operational costs.
Environmental Compliance and Waste Management: The production of acetazolamide generates waste products such as sulfur residues, solvents, and process effluents. Proper waste management systems must be implemented to comply with environmental regulations. Investing in waste treatment technologies is critical to minimize the environmental impact and ensure sustainable production.
By optimizing procurement resources and investing in modern production technologies, manufacturers can streamline the acetazolamide production process, improve yields, and reduce overall costs.
Acetazolamide (Diamox): An Overview
Acetazolamide, sold under the brand name Diamox, is a sulfonamide-based drug that acts as a carbonic anhydrase inhibitor. It is widely used in clinical settings to manage various conditions, including:
Glaucoma: Acetazolamide reduces intraocular pressure by decreasing the production of aqueous humor in the eye, making it an effective treatment for both open-angle and secondary glaucoma.
Altitude Sickness: Acetazolamide is commonly prescribed to prevent and treat acute mountain sickness. It helps acclimatize the body to high altitudes by improving respiratory function and reducing fluid retention.
Epilepsy: Acetazolamide has been used as an adjunct therapy for certain types of epilepsy, particularly in cases of absence seizures. Its ability to alter ionic balances in the brain contributes to its antiepileptic effects.
Edema: As a diuretic, acetazolamide promotes the excretion of bicarbonate, sodium, and water, making it effective in treating edema caused by congestive heart failure or drug-induced fluid retention.
Given its wide range of applications, acetazolamide is a vital medication in both acute and long-term therapeutic settings.
Market Drivers
Several key factors drive the demand for acetazolamide in the global pharmaceutical market. These market drivers include:
Increasing Prevalence of Glaucoma: The rising incidence of glaucoma, particularly in aging populations, is a major driver of demand for acetazolamide. As glaucoma remains a leading cause of blindness worldwide, the need for effective intraocular pressure management solutions, such as acetazolamide, continues to grow.
Rising Popularity of Outdoor Activities and Travel: With more people engaging in high-altitude outdoor activities such as mountaineering, trekking, and skiing, the demand for acetazolamide as a preventive treatment for altitude sickness has increased. Additionally, with more travelers visiting high-altitude destinations, healthcare providers are increasingly prescribing acetazolamide for safe acclimatization.
Expanding Epilepsy Treatment Options: Although newer antiepileptic drugs have emerged, acetazolamide remains a valuable adjunct treatment for certain types of epilepsy, particularly in patients who do not respond well to other medications. The growing demand for diverse epilepsy treatment options continues to support acetazolamide's market presence.
Increased Focus on Edema Management: The rising prevalence of conditions such as heart failure and chronic kidney disease, both of which can lead to edema, is driving the demand for diuretics like acetazolamide. Its role in managing fluid retention makes it an essential part of the therapeutic regimen for patients with these conditions.
These market drivers indicate a stable and growing demand for acetazolamide, making it a valuable pharmaceutical product in the global market.
Raw Materials Requirements
The production of acetazolamide requires several high-quality raw materials to ensure product purity and efficacy. The primary raw materials involved in the production process include:
Sulfonamide Precursors: The synthesis of acetazolamide relies on sulfonamide derivatives as key intermediates. These compounds form the backbone of the final product and must be sourced from reliable suppliers to ensure high purity.
Acetic Acid: Acetic acid is used as a reagent in the condensation reaction that forms the acetazolamide molecule. It acts as both a solvent and a reactant, making it a critical component in the synthesis process.
Ammonia: Ammonia is involved in the formation of key functional groups within the acetazolamide structure. Its role in the chemical reactions is essential for producing the desired pharmacological properties.
Organic Solvents: Solvents such as acetone, ethanol, and methanol are used during the synthesis and purification stages of acetazolamide production. These solvents help dissolve raw materials, facilitate chemical reactions, and purify the final product.
Water: High-purity water is required for the preparation of solutions, rinsing equipment, and crystallization of the final product. The quality of water used in pharmaceutical production is strictly regulated to prevent contamination.
Ensuring a consistent and high-quality supply of these raw materials is crucial for maintaining production efficiency and meeting regulatory requirements.
Costs and Key Process Information
The cost structure of acetazolamide production is influenced by several factors, including raw material prices, energy consumption, labor, and capital investment. Key cost components include:
Raw Material Costs: The price of sulfonamide precursors, acetic acid, and ammonia can vary depending on global market conditions. Establishing long-term contracts with suppliers can help stabilize raw material costs and reduce the risk of price fluctuations.
Energy Costs: The production of acetazolamide involves several energy-intensive steps, particularly during chemical reactions and purification. Energy costs can be a significant factor in the overall production cost, making energy efficiency a key consideration for manufacturers.
Labor and Operating Costs: Skilled labor is required to operate the reactors, monitor the synthesis process, and ensure product quality. Labor costs vary depending on the level of automation in the facility and the geographical location of the production site.
Capital Expenditures: The production of acetazolamide requires significant capital investment in specialized equipment, such as reactors, filtration systems, and crystallization units. Automated systems can reduce labor costs and improve production efficiency, but they require a higher initial investment.
Regulatory Compliance Costs: Acetazolamide is a pharmaceutical product, and its production must comply with strict regulatory standards, including Good Manufacturing Practices (GMP). Meeting these regulatory requirements involves additional costs for quality control, testing, and validation.
By optimizing these cost factors and investing in modern production technologies, manufacturers can improve the efficiency and profitability of the acetazolamide production process.
Looking for an Exhaustive and Personalized Report to Substantiate Your Business?
For businesses seeking a detailed analysis of the acetazolamide production process and market dynamics, a personalized and exhaustive report can provide the necessary insights to support strategic decision-making. A customized report can include:
In-Depth Cost Breakdown: A comprehensive analysis of production costs, including raw material procurement, energy consumption, labor, and capital investments, tailored to your specific production facility.
Market Demand Projections: Insights into future demand for acetazolamide in key therapeutic areas such as glaucoma, altitude sickness, and epilepsy, allowing you to plan for capacity expansions and market entry strategies.
Regulatory Compliance and Quality Control: Guidance on meeting regulatory requirements, ensuring product safety, and maintaining high standards in the production of pharmaceutical-grade acetazolamide.
Supply Chain Optimization: Recommendations for sourcing high-quality raw materials, managing procurement logistics, and ensuring a reliable supply chain to meet growing demand.
Partnering with industry experts to develop a comprehensive, data-driven report can help your business navigate the complexities of the acetazolamide market, enhance production efficiency, and ensure long-term success.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Amanda Williams
Email: [email protected]
Toll-Free Number: USA Canada – Phone no: +1 307 363 1045 | UK – Phone no: +44 7537 132103 | Asia-Pacific (APAC) – Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA